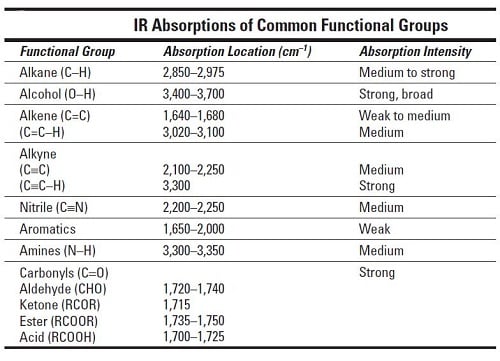
It is well known that all molecules chemicals have distinct absorption regions in the IR spectrum. The assignments of IR bands of surface-activated species are summarized in Table S3.

Using Reference Materials Part I Standards For Aligning The X Axis
How To Find Functional Groups In The Ir Spectrum Dummies
Science Skool Infrared Spectroscopy
3 Absorbance of 001 M Cr.
Ir absorbance spectrum table. Two other regions of the IR spectrum can quickly yield useful information if you train yourself to look for them. This technique works almost exclusively on samples with covalent bonds. Spectrophotometry uses photometers known as spectrophotometers that can measure the intensity of a light beam at different wavelengthsAlthough spectrophotometry is most commonly applied to.
If there is a problem contact the manufacturer. There are two tables grouped by frequency range and compound class. If it was determined by absorbance at 280 nm or a dye binding technique it could be too low.
To identify the white substance a spectrum from a clean area of the surface was taken as a reference a Ge-crystal was used being it ideal for such spectroscopic task. Identification of Cell Culture Media with Raman Spectroscopy. Le spectre visible est la partie du spectre électromagnétique qui est perceptible par lhumainLe spectre se décompose en rayonnements monochromatiques par le passage de la lumière à travers un dispositif disperseur prisme ou réseau diffractant.
Light visible to the human eye occupies only a small portion of the electromagnetic spectrum namely from about 350 to 750 nanometers or from violet to red. 3 Monochromatic transmittance T and absorbance A of radiance along the path are defined as T exp A 1 T 1 exp 72 NOTE. If the isoprene spectrum on the right was obtained from a dilute hexane solution c 4 10-5 moles per liter in a 1 cm sample cuvette a simple calculation using the above formula indicates a molar absorptivity of 20000 at the maximum absorption wavelength.
Check the concentration of the sample. Calculating Your Working Tolerance. The instrument used in ultravioletvisible spectroscopy is called a UVVis spectrophotometerIt measures the intensity of light after passing through a sample and compares it to the intensity of light before it passes through the sample The ratio is called the transmittance and is usually expressed as a percentage TThe absorbance is based on the transmittance.
An absorption spectrophotometer is a device used to measure absorbed light intensity as a function of wavelength. The signal to noise is very low. Table 1 as photosensitizer E 0 Irppy 3 Irppy 3.
For systematic evaluation the IR spectrum is commonly divided into some sub-regions. Conventionally the IR region is subdivided into three regions near IR mid IR and far IR. Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength.
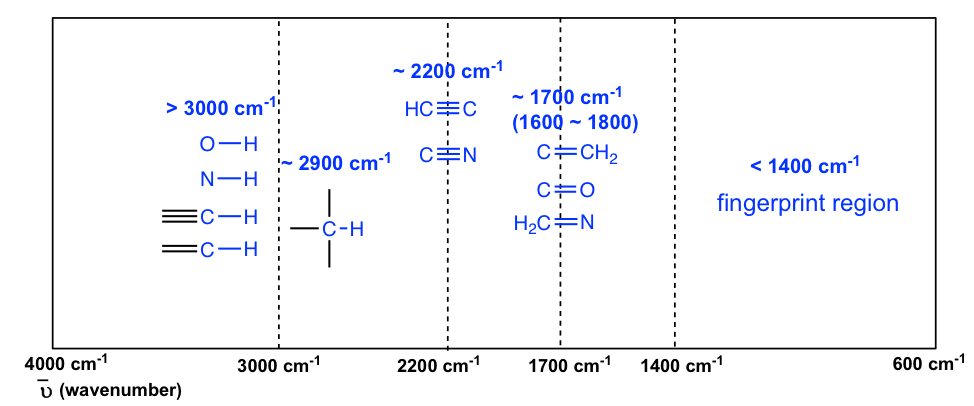
The IR region of the electromagnetic spectrum ranges in wavelength from 2 -15 µm. The line at 3000 cm -1 is a useful border between alk ene CH above 3000 cm -1 and alk ane CH below 3000 cm -1 This can quickly. Transmission function Transmittance NOTE.
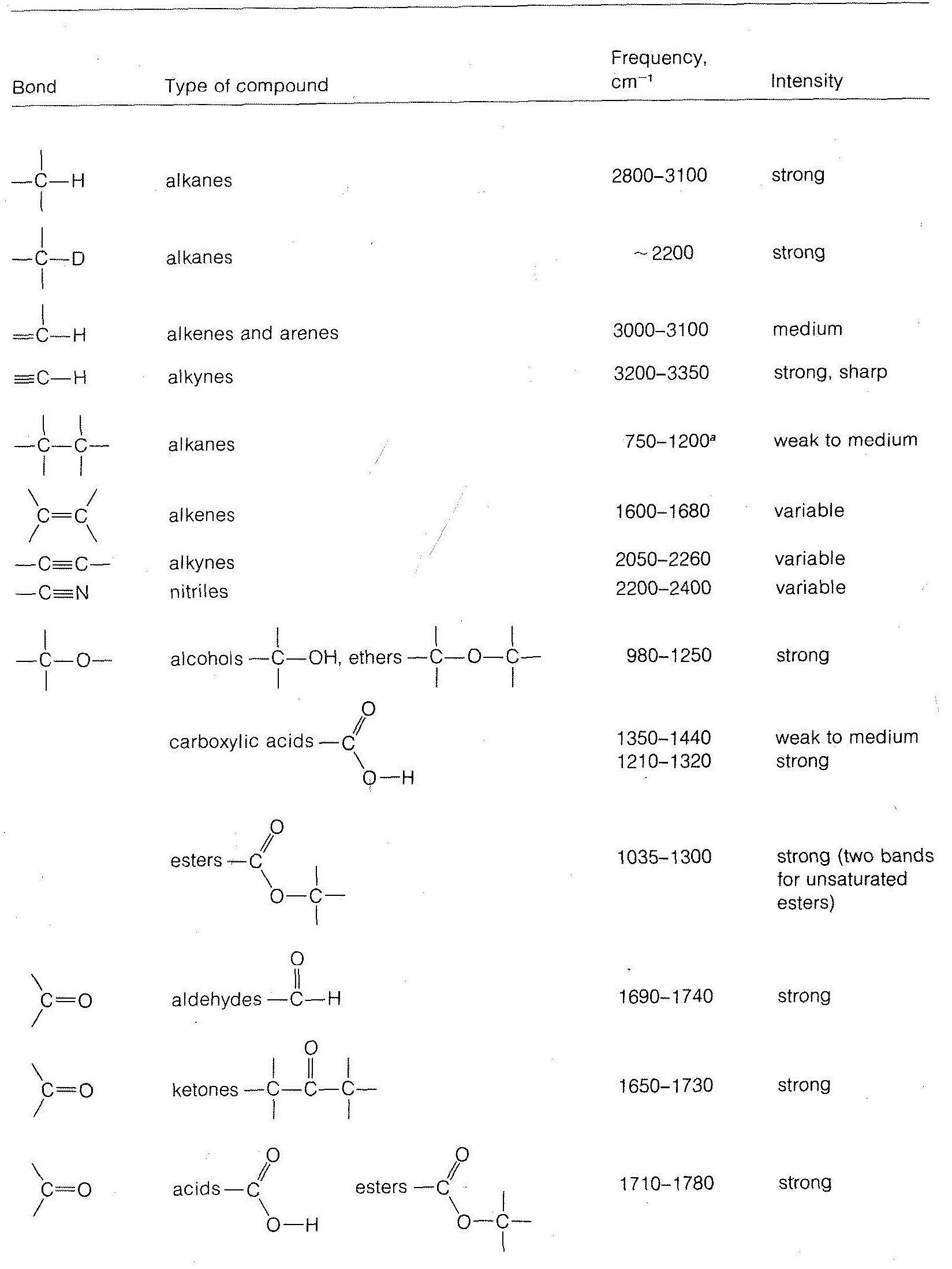
A is also called absorption or absorption function or absorptivity. Gaseous absorption in the IR Main atmospheric gases absorbingemitting in the IR. The table lists IR spectroscopy frequency ranges appearance of the vibration and absorptions for functional groups.
Due to the low energy of infrared IR rays microwaves and radio waves they are not harmful to living ones. 3 was taken at nine different wavelengths from 400 to 600 nm. A strong absorbance on the left towards the UV and a weak absorbance on the right towards the visible.
Wavelength Absorbance of 003 M Cr. The absorption bands in IR spectra have different intensity. Widely used in both research and industry infrared spectroscopy is a simple and reliable technique used for a variety of.
Table PageIndex7 shows the absorption frequencies of common types of functional groups. UV violet blue green yellow red IR. Table 1 summarizes reports of IR radiation being used to interact with cells and tissues.
Note how it is qualitatively similar to that of acetone. Simple spectra are obtained. Our optical spectrum analyzers acquire the spectrum via Fourier transform using a scanning Michelson interferometer.
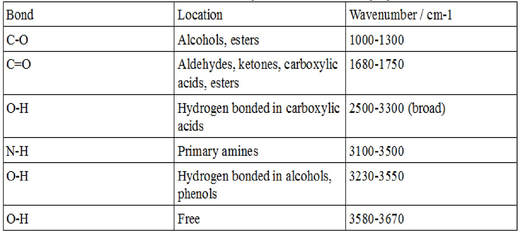
Table 61 Characteristic IR Frequencies of Stretching Vibrations. It was found that the absorbance of PDA-3coated CM was 94 and 88 in the UV 200 to 400 nm and visible 400 to 780 nm regions respectively. The table below indicates the IR spectral regions.
Absorbance spectrum can be produced showing at which IR wavelengths the sample absorbs. This approach enables a high-precision Wavelength Meter mode with seven significant figures and 1 part-per-million ppm spectral precision allows robust statistical analysis of the acquired spectra and provides broadband spectral measurements with every scan. The information in Table 61 can be summarized in the diagram that is easier to be identified Figure 63b in which the IR spectrum is divided in several regions with the characteristic band of certain groups labelled.
It also highlights some of the medical applications of IR radiation. Figure 63b Approximate IR Absorption Range. Figure 6 shows a black rubber with white crystalline substance on the surface.
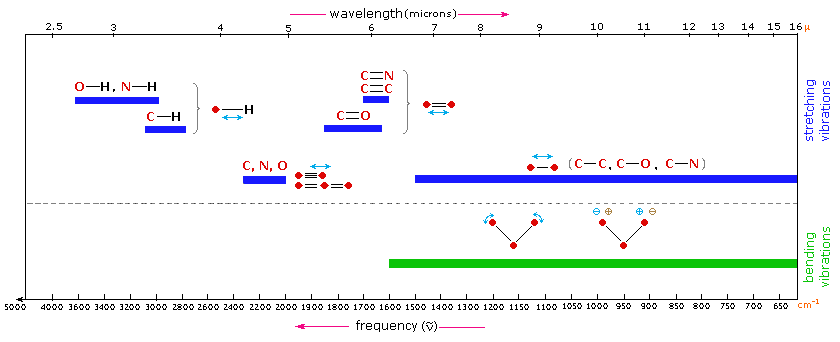
Below is a table of the data followed by the absorbance spectrum. Organic chemists loosely refer to wavenumbers as frequency and so in books and other sources you may see spectra labelled as frequency cm-1. La sensibilité de lœil selon la longueur donde diminue progressivement de part et dautre dun maximum entre.
Therefore the PDA-3based device could absorb majority of solar energy across the main spectrum. The IR Spectrum Table is a chart for use during infrared spectroscopy. 2 Check the spectrum of air to make sure the lamp is stable and the baseline is not drifting.
There is very low ellipticity. Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about the sample is in. Most of the IR used originates from the mid IR region.
In UVvisible spectrophotometers a beam of light from a suitable UV andor visible light source is passed through a prism or diffraction grating monochromatorThe light then passes through the sample to be analyzed before reaching the detector Fig. In contrast to acetone however where the weak absorbance is at 260 nm the weak absorbance in Aniline Yellow is in the visible region of the spectrum at about 460 nm. Colour Spectrum is a physical continuum of light and the mixture of all colours of different wavelengths in the visible region is known as white light.
The absorbance spectrum of an aqueous solution of chromium III ion Cr. Generally rubber has a strong IR-absorbance due to embedded carbon black particles. A transmittance to absorbance table enables fast conversion from transmittance values to absorbance in the lab or in the field.
Data Table WAVELENGTH nm ABSORBANCE Chlorophyll a Chlorophyll b Carotenoids 360. IR fingerprint regions of 12001500 cm-1 19002100 cm-1 and 30003050 cm-1 are concerned in which the characteristic peaks of the intermediates are usually observed. Analysis of these absorption characteristics reveals details about the molecular structure of the sample.
The wavelengths of the light sources are proposed to match the absorption spectrum of CCO or water molecules. The spectrum was determined for two solutions of different concentrations. Using electron donors with high reducing ability should thus be favourable and adding 02 mM of Irppy 3 4 where ppy is phenylpyridine.
Infrared Spectroscopy
9 8 Infrared Rovibrational Spectroscopy Chemistry Libretexts
Infrared Spectroscopy Part 1 Laboratory Manual
How Can I Distinguish Functional Group Region And Fingerprint Region In A Infrared Spectrum Socratic
Utdallas Edu
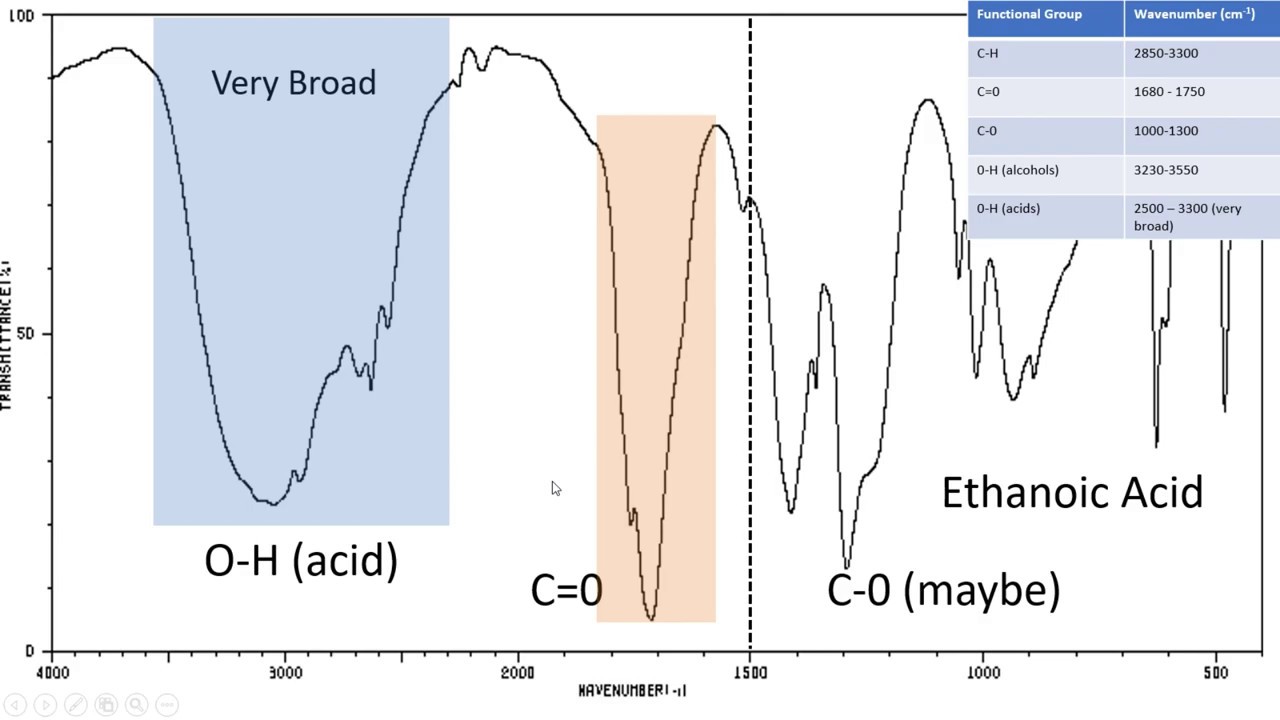
Introduction To Ir Spectroscopy How To Read An Infrared Spectroscopy Graph Youtube

6 3 Ir Spectrum And Characteristic Absorption Bands Organic Chemistry
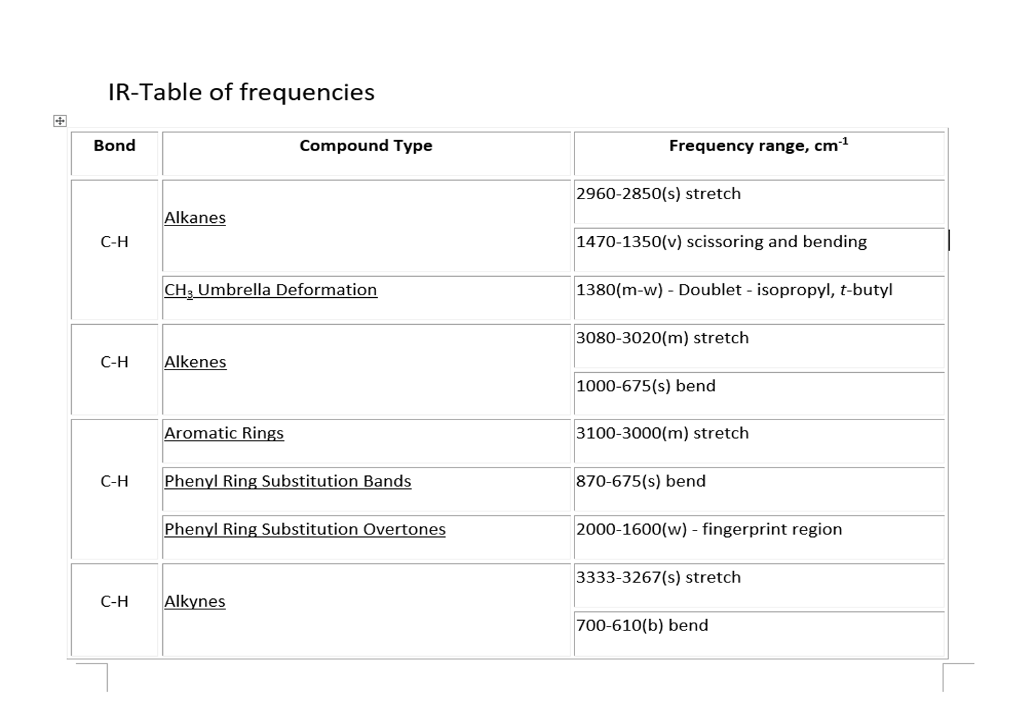
Solved Ft Ir Spectroscopy Refer To The Ir Frequency Chart Chegg Com
