If the transmittance is 100 what would be the absorbance. So it only measures the Transmittance.

How To Interpret Ir Spectra Chemistryscore
How Can I Distinguish Functional Group Region And Fingerprint Region In A Infrared Spectrum Socratic
Introduction To Ir Spectroscopy How To Read An Infrared Spectroscopy Graph Youtube
It is a member of the International Science Council ISC.

Ir absorbance table. There have been many advances in the field of IR Spec the most notable was the application of Fourier Transformations to this technique thus creating an IR method that had higher resolution and a decrease in noise. Transmission function Transmittance NOTE. In fact the isotopic abundance of 35 Cl and 37 Cl may be calculated from the relative absorbance values in the IR spectrum since absorbance is proportional to concentration.
Karl Norris started using IR Spectroscopy in the analytical world in the 1960s and as a result IR Spectroscopy became an accepted technique. 3 was taken at nine different wavelengths from 400 to 600 nm. Organic chemists loosely refer to wavenumbers as frequency and so in books and other sources you may see spectra labelled as frequency cm-1.
Energy near-IR approximately 14000-4000 cm-1. The editorial Board of the Journal of Renewable Energy and Environment- JREE is pleased to announce that JREE is accepted to be indexed by Scopus. If the absorbance is 2 what would be the transmittance.
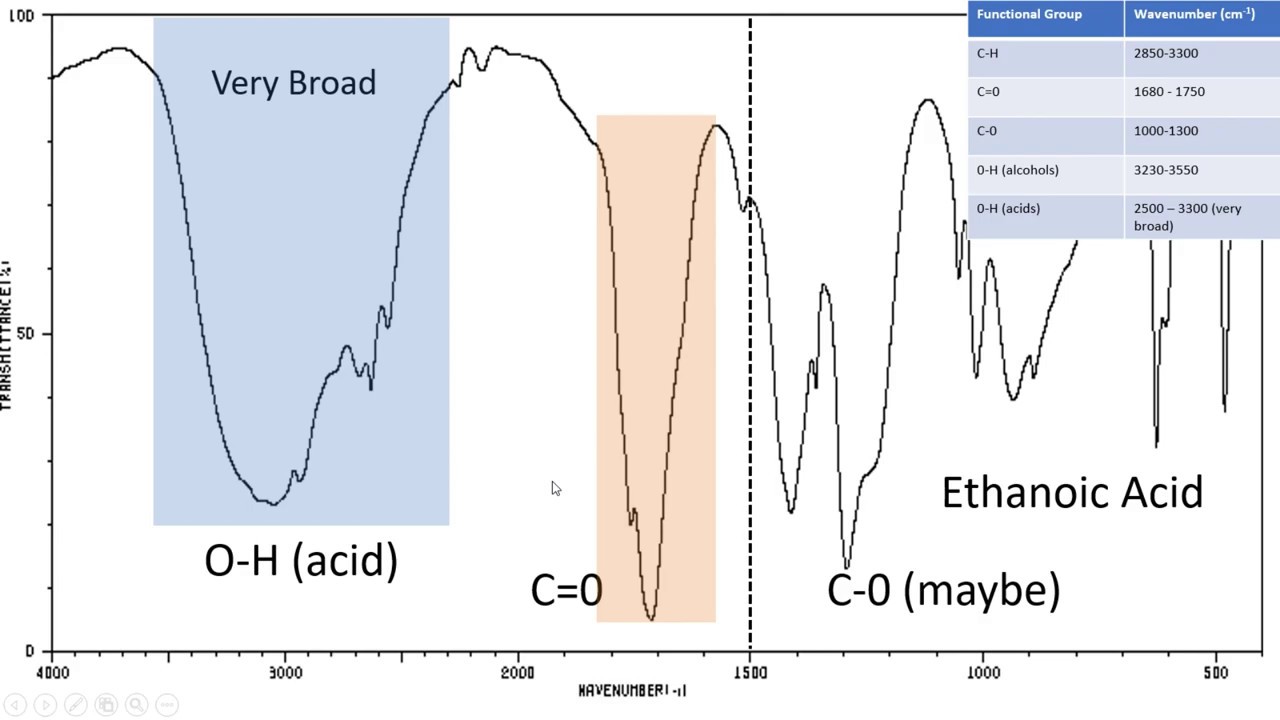
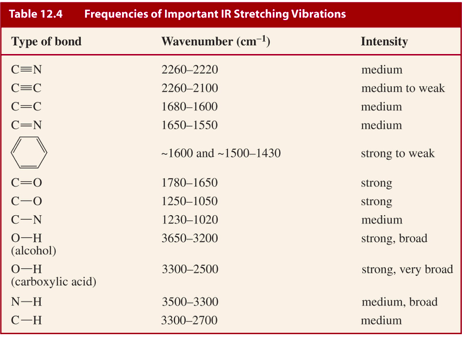
The absorbance spectrum of an aqueous solution of chromium III ion Cr. The following table provides a collection. Table PageIndex7 shows the absorption frequencies of common types of functional groups.
Figure 63b Approximate IR Absorption Range. Table 61 Characteristic IR Frequencies of Stretching Vibrations. If the isoprene spectrum on the right was obtained from a dilute hexane solution c 4 10-5 moles per liter in a 1 cm sample cuvette a simple calculation using the above formula indicates a molar absorptivity of 20000 at the maximum absorption wavelength.
Spectrophotometry uses photometers known as spectrophotometers that can measure the intensity of a light beam at different wavelengthsAlthough spectrophotometry is most commonly applied to. The springs vibrate and each one sings at a characteristic frequency which depends on the strength of the bond and on the masses of the. The FluoroMax series represents HORIBAs industry-leading fluorometer performance in a convenient affordable easy-to-use benchtop model.
Indeed the entire vertical absorbance scale may be changed to a molar absorptivity scale once this information about the sample is in. Use this chart as a handy reference in the lab classroom or field. 3 Absorbance of 001 M Cr.
There are two tables grouped by frequency range and compound class. The absorbance and transmittance are related to each other. The FluoroMax family with its unique all reflective optics and photon counting was the first to bring the sensitivity of a modular fluorometer to a tabletop fluorescence instrument.
Widely used in both research and industry infrared spectroscopy is a simple and reliable technique used for a variety of. Absorbance at 254 nm and 1 em optical path length 0001 OQ1 Not specified Sub-clause 74 absorbance units max. Transmittance to absorbance table for fast conversion.
In UVvisible spectrophotometers a beam of light from a suitable UV andor visible light source is passed through a prism or diffraction grating monochromatorThe light then passes through the sample to be analyzed before reaching the detector Fig. Journal of Renewable Energy and Environment is Accepted to be indexed by Scopus. 001 NOTES 1 Because of the difficulties associated with measurement of the pH.
Wavelength Absorbance of 003 M Cr. Absorbance near 205 nm and a less intense peak in 255-275 nm range. Silica Si02 content mg1 max.
Though the change of an isotope eg 35 Cl to 37 Cl does not effect the equilibrium bond length r e or the force constant k for the molecule varying an isotope does change m the reduced mass. For instance the absorbance of the alkene 2-methyl pent-2-ene is below 200 nm as is the ππ absorbance of 4-methyl pentane-2-one below. The spectrum was determined for two solutions of different concentrations.
To identify the white substance a spectrum from a clean area of the surface was taken as a reference a Ge-crystal was used being it ideal for such spectroscopic task. The online transmittance chart is available to download and share. The table lists IR spectroscopy frequency ranges appearance of the vibration and absorptions for functional groups.
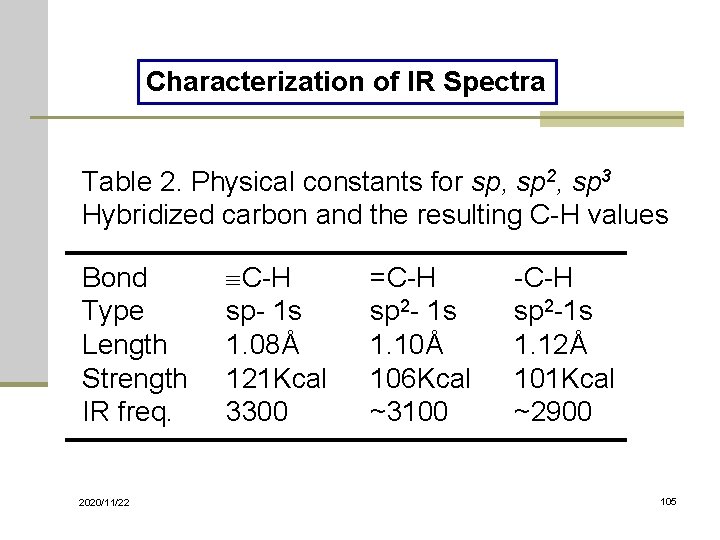
The 2 Most Important Things To Look For Tongue and Sword Last post we briefly introduced the concept of bond vibrations and we saw that we can think of covalent bonds as a bit like balls and springs. Spectrophotometry is a branch of electromagnetic spectroscopy concerned with the quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. The absorption bands in IR spectra have different intensity.
EQUIPMENT AND MATERIALS NEEDED 2 or 3 fresh spinach leaves wooden ruler 600 mL beaker plastic wrap chromatography paper or filter paper pencil copper penny coin 100 mL graduated cylinder 5 test tubes scissors stapler. An absorption spectrophotometer is a device used to measure absorbed light intensity as a function of wavelength. JREE is a peer-reviewed open access journal published jointly by Materials and Energy Research Center-MERC and Iranian Association of Chemical Engineering-AIChE.
Below is a table of the data followed by the absorbance spectrum. Absorbance A is the logarithm to the base 10 of the reciprocal of the transmittance T. Gaseous absorption in the IR Main atmospheric gases absorbingemitting in the IR.
The old IR-spectra can only be found in Transmittance mode. 3 Monochromatic transmittance T and absorbance A of radiance along the path are defined as T exp A 1 T 1 exp 72 NOTE. How To Interpret IR Spectra In 1 Minute Or Less.
IUPAC is registered in Zürich Switzerland and the administrative office known as the IUPAC Secretariat is in Research. A is also called absorption or absorption function or absorptivity. Generally rubber has a strong IR-absorbance due to embedded carbon black particles.
Analysis of these absorption characteristics reveals details about the molecular structure of the. Absorbance spectrum can be produced showing at which IR wavelengths the sample absorbs. Substitution and increased conjugation moves the absorbance to longer wavelengths 1H NMR.
Aromatic Hs strongly deshielded by ring and absorb between δ 65 and δ 80 Peak pattern is characteristic positions of substituents 30 C 8H 9Br C 9H 12 2H 2H 2H 3H 4H 2H 3H 3H. The information in Table 61 can be summarized in the diagram that is easier to be identified Figure 63b in which the IR spectrum is divided in several regions with the characteristic band of certain groups labelled. We would like to show you a description here but the site wont allow us.
The use of absorbance and transmittance is a personal preference. But after the use of microprocessors it automatically converts transmittance into absorbance. In mesityl oxide where the alkene and CO group are in conjugation with each other the absorption maximum moves to longer wavelength at 228 nm.
The International Union of Pure and Applied Chemistry IUPAC ˈ aɪ juː p æ k ˈ juː- is an international federation of National Adhering Organizations that represents chemists in individual countries. Residue after evaporation Not applicable 1 2 Sub-clause 75 on heating at 110 C see note 3 mgkg max. Figure 6 shows a black rubber with white crystalline substance on the surface.
It is well known that all molecules chemicals have distinct absorption regions in the IR spectrum. Steady State and Lifetime Benchtop Spectrofluorometer. The IR Spectrum Table is a chart for use during infrared spectroscopy.
The antioxidant values of foods listed are expressed in ORAC Oxygen Radical Absorbance Capacity units a unit of measurement for antioxidant content which was originally developed by the National Institute on Aging at the National Institutes of Health You can browse foods alphabetically to find their ORAC values or if you want to get straight to the best antioxidants and purported anti.

Ftir Frequency Range And Functional Groups Present In The Sample After Download Table

Infrared Spectroscopy Protocol

The Absorption Peaks In Ft Ir Spectrum And Their Assignment Of Compound Download Table
Solved Refer To The Ir Spectra Of Pure Dibenzalacetone And Chegg Com

The Ir Spectrum Of Benzoic Acid Is Include Clutch Prep

Ir Spectroscopy Functional Groups Ir Absorbance Table Http Www Chromatographytechniques Com Articles Wissenschaft Wunsche
2
Spectroscopy Infrared Spectroscopy Introduction N Spectroscopy Is An
